
Simply shaking the two liquids produces unstable emulsions. The process is improved by homogenizing a mixture of the two liquids using a colloid mill. Agitating a small part of one liquid with the majority of the other can accomplish this.
Emulsion surface chemistry free#
Acid changes soap into soluble free fatty acids that cannot be dissolved.Įmulsification is the process of dispersing a liquid in the form of an emulsion. A strong acid, for instance, can be added to break up an oil-water emulsion that has been stabilized by soap. By eliminating the emulsifying agent, they are also broken.

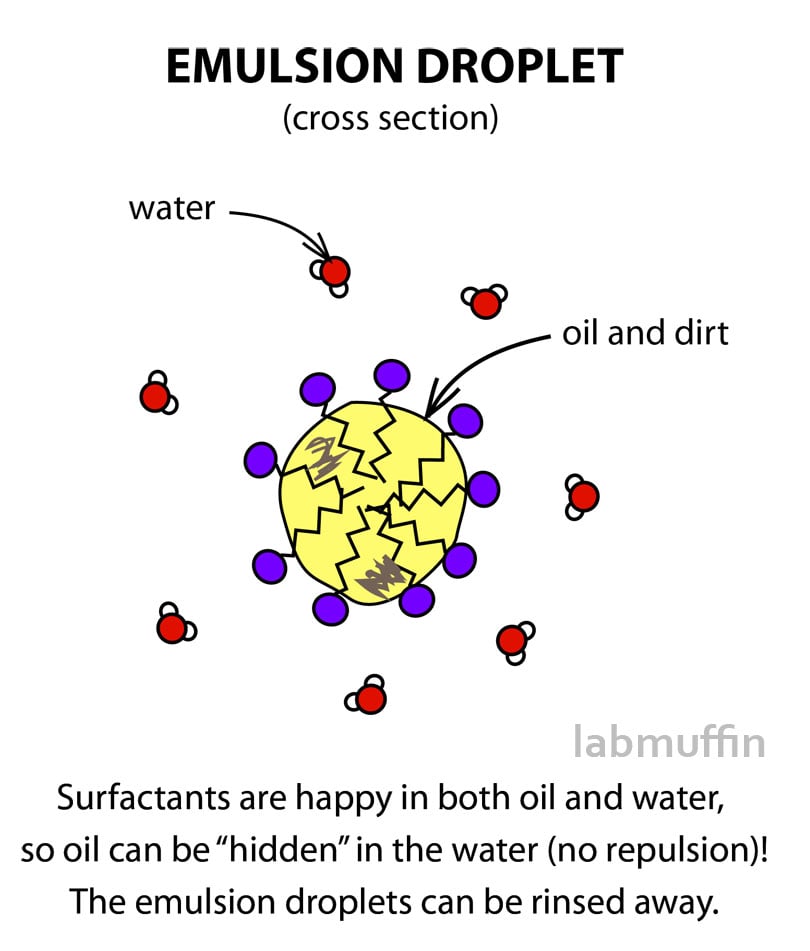
Demulsification: Emulsions can be “demulsified”-broken into their component liquids-by heating, centrifuging, freezing, or by adding significant amounts of electrolytes.This property of emulsions is used to detect the type of a given emulsion. The dispersed liquid, on the other hand, will instantly create a separate layer when combined with it. Dilution: With any quantity of the dispersion medium, emulsions can be diluted.Butter and cold cream are examples of it. Water-in-Oil type (W/O type): Here, the dispersion medium is oil and the dispersed phase is water.An example of this type is milk, which contains liquid fats that are dispersed in water. Oil-in-Water type (O/W type): Here, water serves as the dispersion medium while oil serves as the dispersed phase.Examples of Emulsionsīutter, milk, Egg yold, cold cream, hair cream, etc. The process of mixing liquid to form an emulsion is called emulsification. Emulsion DefinitionĪn emulsion is defined as a liquid-liquid colloidal system that is formed by combining two or more immiscible liquids. Emulsions are stabilized by substances that either create films on the droplet’s surface, like soap molecules, or give them mechanical stability like colloidal carbon. If the liquids that are mixed have very little or no mutual solubility, emulsions can be formed spontaneously or, more frequently, mechanically via agitation. In most cases, one of the two liquids is water and the other is oil since it is immiscible in water. These are liquid-liquid colloidal systems, however, the two liquids are immiscible. An emulsion is a dispersion of finely divided liquid droplets in another liquid.


 0 kommentar(er)
0 kommentar(er)
